The properties of water
It is therefore important to review the suppression potential of water and how it is used to control and extinguish fire.
|
Suppression Potential: The potential of removing heat or energy from the fire. |
Water is applied in a liquid form to the fire and is vaporized into steam. Imagine that we place 1 L of water, in the form of droplets, into a hot smoke layer to cool it and change its flammability. Smaller droplet size, and therefore increased total surface area of the droplets, speeds heat absorption and increases the efficiency of the applied water.
Three physical properties of water affect how heat energy is absorbed:
- Specific heat of water;
- Latent heat of vaporization; and
- Specific heat of steam.
Specific Heat of Water
A known amount of energy is required to raise the temperature of a known mass of water. This value is known as the specific heat of water. When water is used to cool fire gases (i.e., smoke), water droplets are placed in the smoke layer. Energy will be transferred from the smoke to the cold water droplets until the water reaches a temperature of 100°C (212°F). This value is 4,186 J/kg°C (approximated to 4.2 kJ/kg°C), as shown in Figure 16.
Latent Heat of Water (Vaporization)
Water will absorb considerably more energy from smoke as the hot water vaporizes to steam. The rate that energy absorbed is during this change of state (liquid to vapour) is known as the latent heat of vaporization, and it has a value of 2,260 kJ/kg.
Specific Heat of Steam
When the steam is dispersed into the smoke layer, more energy will be absorbed from the smoke layer. The result will be a rise in the steam's temperature. This energy absorption is known the specific heat of steam. This value is 2,080 J/kg°C (approximated to 2.1 kJ/kg°C)
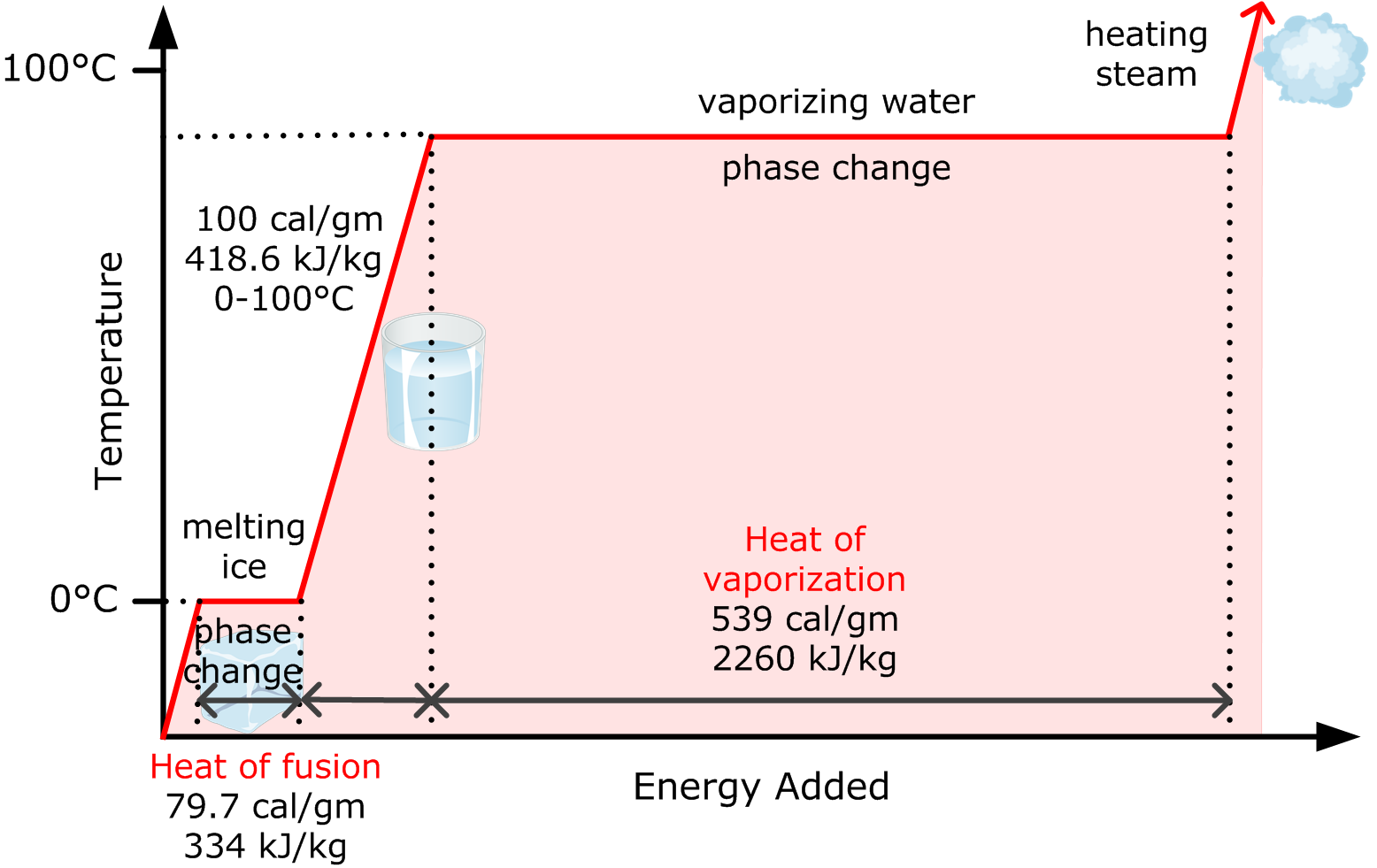
When water is used to absorb heat from a fire, this normally results in both a change of temperature and a change of state.
To calculate the heat required to raise the temperature of water, the following equation is used:
Heat absorbed = mass (kg) x specific heat x temperature change
The following equation is used to calculate the heat absorbed during a change of state:
Heat absorbed = mass (kg) x latent heat
Example Calculation
Water emerges from a nozzle at 10°C and is converted by the heat from the fire into steam at 200°C. If 1 L (1 kg) of water is applied, how much heat is removed from the smoke layer? Remember that:
- Specific heat of water = 4.2 kJ/kg°C
- Latent heat of vaporization of water = 2260 kJ/kg
- Specific heat of steam = 2.0 kJ/kg°C
This calculation must be made in three steps:
- Calculate the heat absorbed to raise the temperature of water from 10°C to 100°C:
Q1 = 1 x 4.2 x 90 = 378 kJ - Calculate the heat absorbed to change water at 100°C to steam at 100°C:
Q2 = 1 x 2260 = 2,260 kJ - Calculate the heat absorbed to raise the temperature of the steam at 100°C to 200°C:
Q3 = 1 x 2.0 x 100 = 200 kJ
The amount of heat removed from the smoke layer is:
Total heat = Q1 + Q2 + Q3 = 2,838 kJ
Therefore, 1 L of water sprayed onto the fire removes about 2,838 kJ of heat. A nozzle at 570 L/min (150 gpm) delivers 1 kg (2.2 lbs) of water in slightly under a tenth of a second.
Both specific heat and latent heat are properties of a given material. In other words, every time the material is heated or cooled-no matter how quickly or by what heating process-the same amount of heat energy is transferred to reach the same state.
In the sample calculation above, the energy required to heat water from 10°C to the boiling point of 100°C is 378 kJ. This is very little in comparison to the energy required to evaporate the boiling water, which is 2,260 kJ.
If heating 1 L of water to its boiling point and converting that water to steam represents a total suppression capacity of 2,638 kJ/kg, we see that simply warming the water to 100°C without converting it to steam is, at most, only 14% as efficient. Therefore, most water on the floor of a fire scene has, at best, been used to 14% of its capacity, and was likely less efficient than that. The conversion process from 100°C water into 100°C steam, which is a change in state, absorbs six times more energy than simply heating water until it reaches 100°C.
If we can heat evaporated water even further, we may absorb some extra energy. Heating of water vapour requires 2.0 kJ/kg°C. Thus, steam heated an additional 300°C to 400°C absorbs 600 kJ/kg of additional energy. These properties of water are used regularly, to varying degrees of efficiency, when putting out fires.