Vapour Density
As previously discussed with flaming combustion, a fuel can only ignite when it is in the gaseous or vapour state; therefore, an understanding of gaseous combustion is essential to predicting how fires will start and spread, no matter what fuel is involved. If the fuel starts as a gas, the fire may burn with either premixed or diffusion flames. When a fire interacts with liquid or solid fuels, the vapours most often burn as diffusion flames since there is little time for mixing of fuel and air before the reaction starts.
Gaseous combustion depends on the concentration of fuel in the air, how the fuel is contained in a space, and the type of fuel that is present. The vapour density of a gas refers to its density compared to air. Gases that have a vapour density of around 1 will mix evenly with the air. A gas with a vapour density greater than 1 is heavier than air. It will sink and can collect in low areas such as drains and trenches, filling a compartment from the floor upwards. Gases that have vapour densities less than 1 are lighter than air and will rise to the top of a compartment, possibly collecting in attic and overhead areas.
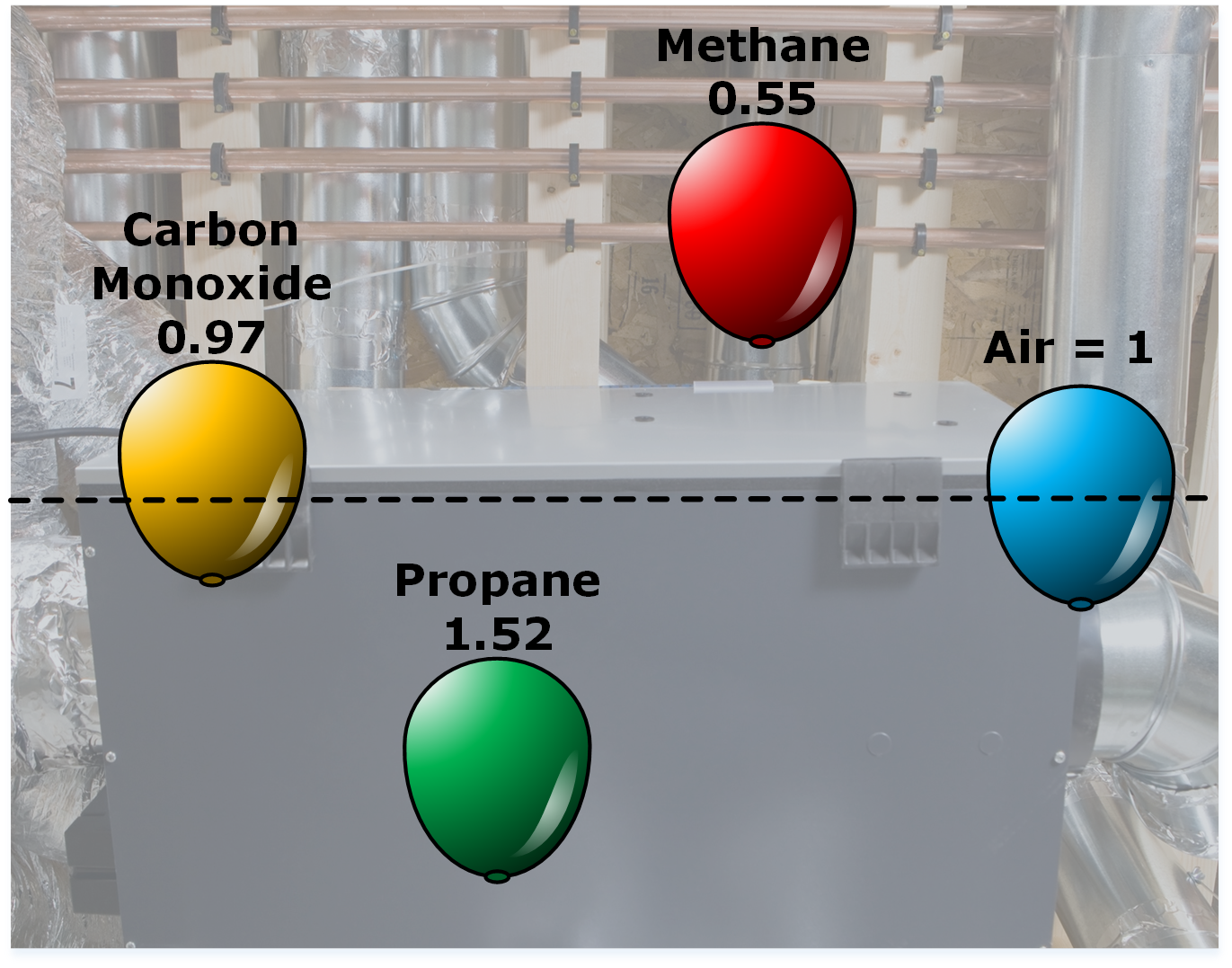
When we are dealing with the presence of gases during a fire event, we have to be able to identify what types of gases we might be dealing with and then determine where they might collect and what possible dangers they present.